The ultimate goal of the research center is to make an impact by designing efficient routes for storing electrical energy in the form of chemical bonds, i.e. in chemical compounds that can be used either as energy carriers, or as building blocks for chemical production.
Each of the processes under consideration lack suitably efficient catalysts; without such catalysts, these processes are unable to make a significant impact.
The centre will develop a systematic methodology to accelerate the discovery process for new catalysts. It will do that in a concerted effort, which is composed of six interdependent sub-projects.
The six sub-projects are closely interlinked; the methods and insight gained from each will support the others.
|
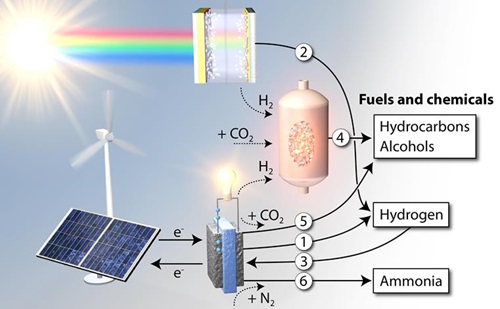
Figure: The role of the six sub-projects in the conversion of sustainable energy to fuels and chemicals
|
1. Efficient electrolytic water splitting for hydrogen production.
Background:
The simplest solar fuel is hydrogen, which can be formed by the electrolysis of water. The hydrogen can be compressed and used in fuel cells as a zero emission fuel for the transportation sector.
Challenge:
There are still enormous challenges associated with the catalysis of water splitting. The best known catalysts have substantial potential losses (overpotentials) and are not sufficiently stable.
Responsible: Tejs Vegge
2. Direct harvesting of sunlight for hydrogen production
Background:
Instead of relying on PVs or wind turbines to produce electricity for the electrolytic production of sustainable hydrogen, one could integrate the photon absorption and the catalysis in a photo-electrochemical device for water splitting.
Challenge:
There is both a challenge of finding good catalysts and additional challenges associated with finding suitable solar photon absorbers that can be interfaced with the catalysts.
Responsible: Karsten W. Jacobsen
3. Fuel cell processes for electricity generation
Background:
Hydrogen and a number of simple chemicals such as methanol can be used in fuel cells to generate electricity. Hydrogen-based fuel cells remain attractive for the transportation sector, and several car manufacturers have launched fuel cell-based cars to the commercial market in 2015; Toyota Mirai is the most notable of these.
Challenge:
The catalysis associated with the oxygen reduction processis very inefficient. The most active catalysts are based on Pt: even so, copious loadings of the precious metal are needed, because it has a large overpotential.
Responsible: Jan Rossmeisl
4. Thermally driven processes for CO2 reduction to fuels and chemical building blocks
Background:
Despite the numerous advantages of hydrogen as a fuel, its storage is somewhat problematic; in that context liquid fuels, especially those compliant with the present fuel infrastructure, would be most desirable. Sustainable production of hydrogen would also enable the synthesis of carbon-based fuels, via the reduction of CO2, to methane, methanol, higher alcohols, or eventually even gasoline or diesel. All can be achieved by thermally driven heterogeneous catalysis. Such a process will also provide a sustainable pathway to base chemicals for the chemical and materials industry.
Challenge:
Find catalysts that will work at low pressure and temperature in distributed units compatible with solar hydrogen production.
Responsible: Anker D. Jensen
5. Electrochemical CO2 reduction to fuels and base chemicals
Background:
An alternative route to produce hydrocarbons and alcohols is through the electrochemical reduction of CO2.
Challenge:
At present no catalysts are sufficiently efficient and stable.
Responsible: Brian Seger
6. Electrochemical N2 reduction to ammonia
Background:
The electrochemical reduction of N2 into ammonia is also a consideration. Ammonia is used primarily as a fertilizer; it is essential for our ever-growing population, and also constitutes an indispensable molecule in the chemical industry. It can also serve as an energy carrier.
Challenge:
No efficient catalyst is known today.
Responsible: Thomas F. Jaramillo